|
| A simple, popular image of the atom, based on Rutherford's nuclear model. The electrons are shown orbiting a nucleus (which is in reality quite small, maybe a marble to a football field, i.e. about 100,000:1). Already, there are complexities, as the electrons (-) and protons (+) attract, so the former are shown in orbit similar to a sun and its planets, but an orbiting charge should give off energy as light, spiralling into the nucleus. This brings us to Bohr's concept of fixed stable orbits with distinct energy levels. And, the nucleus' + charges must mutually repel, so there is a strong, short- ranged nuclear force of attraction that counters this. Neutrons share in this force and help to stabilise the nucleus. The atom is about 3 * 10^-10 m across, and the nucleus, about 2 - 15 * 10^-15 m. That is, the first is about 3/10,000,000,000 th of a metre. (Source: Wiki) |
How can we begin to understand the atom, with a view to first level chemistry?
I guess a good place is back in the 5th - 6th centuries BC, where the idea of an ultimate particle seems to have first come up.
The idea is, take up a rock, a brick or the like. Split it in two, then split a half and keep going. Can this be repeated indefinitely, or is there a final particle that cannot be further cut in parts?
The Greek horror of an actual physical infinity (here, infinitely small . . . ) suggested an answer: no, it has to stop somewhere, with a very small particle, the un-cuttable.
And so, Democritus gave that particle a name: A + TOMOS = not + cuttable.
Ironically, of course, once we discovered an inner structure to what we came to call atoms, and once we discovered radioactivity, it was seen that the ultimate particles of chemical elements like Oxygen, Hydrogen, Carbon, Iron, etc -- could indeed be "cut" by using powerful enough forces.
But the nuclear energies involved are about 1 - 100 million times those we see in chemical reactions or in the emission of visible light.
Leaving off such awesome forces, atoms may give up or gain electrons in orbit -- becoming charged ions -- but have a distinct identity. So, we are able to identify an element as a pure, simple substance with only one kind of atom in it, and we can define the atom as the smallest particle of an element that has its chemical properties. (There are now something like 116 - 118 identified elements, and the number grows as researchers find ways to create new ones using nuclear forces.)
In that context, Chemistry in simple terms is the study of interactions of atoms as they react together by combining, splitting apart, etc.
For instance (using standard letter symbols for types of atoms and dashes to represent chemical bonds between atoms) we can see:
H-H + H-H + O-O --> H-O-H + H-O-H
. . . or, in a more standard way of writing:
2 H2 + O2 --> 2 H2OSimilarly, we can show using a similar conventional "Chemical equation" how hydrochloric acid (HCl) reacts with Sodium Hydroxide (NaOH) to form common salt (i.e. sodium chloride) and water:
H-Cl + Na-O-H --> Na-Cl + H-O-H
. . . or, again in more familiar form:
HCl + NaOH --> NaCl + H2O
The idea here is that, at simple level, electrons orbit the nucleus in rings that "like" to be full -- the "Sun and Planets" model:
The first -- innermost -- orbit, takes just two electrons, and after that the next two take eight. If an orbit is nearly full, the atom will have a strong attractive force and will easily grab one or a few more electrons to fill up the "shell" (becoming a negative ion, an ANION [oopsie, earlier error of memory . . . ]).
If an atom on the other hand has just passed a full shell so it now has one or two electrons in its outermost -- "valence" -- shell these electrons will be partly shielded by the completed shells and will not be so strongly held. Such an atom easily gives up those electrons and becomes a positive ion -- a CATION.
These ionisation-prone atoms tend to combine to form ionic compounds, such as common salt -- as we saw for how hydrochloric acid and sodium hydroxide combine to form sodium chloride and water.
In electrolysis, electrodes pass an electric current through a solution with ions and the cathode (-) attracts cations. The anode (+) attracts anions, often triggering chemical processes such as breaking apart water . . . take a look, tremendous . . . into Hydrogen and Oxygen, etc.
This simple illustration (source) outlines what is going on:
(A "battery" such as we use in cell phones etc, does the reverse, using a chemical reaction to generate an electro-motive force, that can drive a current through an external circuit. Rechargable batteries, of course, can have the reaction reversed by applying a current that undoes the reaction. This can be done for maybe 500 cycles, but then eventually it breaks down the battery and it has to be replaced.)
What about atoms that are in the "middle" of the periodic table , like Carbon?
On this simple view, they can be seen as prone to "share" electrons in pairs with other atoms, forming covalent bonds.
Where Carbon in particular is like a connector-block atom, forming huge molecules based on chains and rings of carbon atoms.
It tends to form four shared bonds, pointing at the corners of a triangle-based pyramid, a tetrahedron. Of course, on paper, this may be shown as a flat sun and planets model, or even more simply as a cross-shape with a C-atom in the middle and four bonded atoms on the arms of the cross, e.g. here with Methane (CH4) -- the main component of natural gas:
This is the basis of organic chemistry [a major branch of Chemistry], and it is the basis for the complex protein, enzyme, carbohydrate and fat molecules used in the living cell.
But what about ionic bonds?
These are "simply" electrostatic. That is positive and negative ions attract, so in solid table salt, the ions form a crystal:
 |
| Three-dimensional bar and ball model of a crystal of NaCl (HT:WebElements ) |
In solution, obviously, the Na and Cl are broken up from the crystal. It turns out that water molecules -- though covalent -- are slightly polarised, with the O being somewhat negative. So, water tends to attract the Na+ and Cl- ions, in effect forming a ball of water molecules around them. This breaks up the crystal and the little clusters of ions surrounded by water molecules move about in solution.
A closer look at the reaction that forms NaCl from hydrochloric acid and sodium hydroxide:
H-Cl + Na-O-H --> Na-Cl + H-O-H
. . . is now in order.
HCl is of course an acid.
In solution -- it can exist as a gas (and is one of the gases erupted by volcanoes) -- it breaks up to form Chloride ions and hydrogen ions, which are going to have the balls of water molecules around them, though it is usual to talk about the H+ going with a single water molecule to form the hydronium ion, H3O+.
Acids are substances that on solution in water, form such H3O+ hydronium ions by releasing protons to the water.
Bases, like NaOH -- and this can be bought as a solid, caustic soda (used in toilet cleaners, caution dangerous) --form ions too. Sodium and OH- ions, hydroxonium ions in this case.
So, when the reaction occurs in solution, all that is really happening is that we form water molecules and leave Na and Cl ions floating in their balls of water molecules. But, of we should now allow this to evaporate or if we should heat it up to drive off the water, the reverse to dissolving happens, and hey presto crystals of NaCl form as the ions attract one another electrically and spontaneously hook up in the crystal structure.
(Many years ago, I did this experiment as a fourth former, and we weighed the resulting salt, which was much as predicted. I don't remember if we snuck a finger tip into the liquid and tasted the salt while the teacher was not watching. (A bit risky as one does not know what might be there as a contaminant. No eating or tasting in and around Chemistry labs is very wise practice. Sniffing, too, needs to be very carefully done -- waft a bit to the nose by holding the mouth of the test tube away from you and gently fanning with hands. And remember, some gases are lethal in very small doses so be very careful to follow safety advice.)
Of course, one consequence of this is that we see that the chemical equation is really an equation that is based on rearranging of atoms, with mass being conserved. The input mass on the left and the output mass on the right will be the same, as atoms are not vanishing into thin air, nor are they popping into existence, they are only being rearranged form one compound to another. Where compounds are molecules made up from bonded groups of diverse atoms, such as H-O-H to make water. (Pure elements also often form molecules, bu the atoms are all of the same kind. In the case of Carbon, there are three main kinds of molecules that are commonly discussed (and a lot of other possibilities): diamonds -- a 3-d tetrahedral structure which is very hard indeed, graphite -- sheets made up from hexagonal clusters of C-atoms that slide over each other, used in pencil leads and as a lubricant, and sixty-atom balls often called "Buckyballs.")
That brings up: how much does an atom or a molecule weigh?
We very nearly get the masses by adding up the masses of protons and neutrons with the much lighter electrons almost negligible (as they weigh in at about 1/1800 of the mass of a proton or a neutron). It turns out thought hat this is a little too much, because there is a special nuclear E = m*c^2 level energy that is taken up from the mass of the protons and neutrons when a nucleus forms. This is known as mass defect or binding energy, and it varies across the periodic table:
This is where atomic energy comes form, by splitting -- fission -- heavy nuclei like Uranium, U, we get energy released, and by joining small ones to make bigger ones -- fusion --we get energy released also. Iron is the peak of the curve and will absorb energy to be split up or fused. (It is believed that stars work by fusing H to form heavier and heavier nuclei, then when a big enough star reaches Iron, Fe, it blows up in a supernova which can be as bright as a whole galaxy of hundreds of billions of stars, for a short time. When that happens, the really heavy elements are formed, and the explosion scatters materials to make new stars and planets.)
The Carbon-12 isotope of Carbon has 6 protons and 6 neutrons, and was chosen as the standard of atomic mass, weighing in at twelve atomic mass units, AMU. One AMU or u -- sometimes called a Dalton or Da -- is therefore:
1.660538921(+/- 73)×10−27 kg
For practical work we usually just use 1.66 or 1.6605 times 10^-27 kg.
(10^-27 is the fraction 1/1 00000 . . . 0 with 27 zeros under the bar. Tiny.)
But, how many atoms of C would we have if we had 12 g of C-12?
ANS: The Avogadro number, 6.02214×1023
This gives us a standard number of molecules or atoms of a given kind or substance, known as the Mole. Hydrogen gas is diatomic weighing in at 2.2 AMU, so 2.2 g of Hydrogen has the same number of molecules. Water has a molecular mass of 18 AMU, pretty nearly. 18 g of water has the same number of molecules. And so forth.
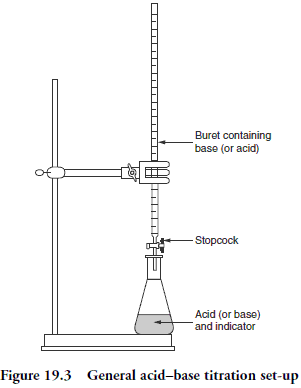
So, given that Chemical equations tell us the number of atoms, ions or molecules that interact to form a new set of substances, we can simply scale up from atoms and molecules etc to Moles by going from p AMU to p grams of the relevant substance etc. And of course, if we dissolve 1 mole of say Na Cl in water, and make up the volume to exactly one litre, we have a solution that is of concentration 1 mol per litre, often called unimolar. This is handy for chemical experiments, which often use solutions in water -- e.g. a titration in which 25 ml of 0.1 molar HCl is dripped out from a burette into a conical flask containing say 25 ml of 0.1 molar NaOH, with maybe an indicator that changes colour dramatically when the solution shifts from basic to acidic. One drop is enough at the end point to trigger that colour shift:
So, we have seen the basis in a nutshell for the chemical view of matter, and for seeing why Chemistry thinks in terms of atoms, ions, molecules, bonds, elements, compounds, reactions and of course acids, bases and salts.
One last thing, how physicists came to accept the general structure of the atom we see today.
In the 1890's, the electron was identified as a particle within the atom that appeared in ion beams and took part in various effects. That meant the atom had in it one or more electrons carrying a negative change and also had a balancing positive charge. That gave rise to the so-called Plum Pudding model with electrons scattered in a pudding of positive charge.
But then between 1909 and 1911, under Rutherford, experiments were done by Geiger and Marsden to shoot alpha particles -- known to be Helium atoms without electrons -- at thin sheets of Gold foil. It was expected that most would go straight through and some would suffer slight deflection. A Zinc Sulphide screen examined by a microscope was used to observe deflected particles, which would give tiny flashes that could be counted and the angle to the beam fired at the foil could be measured.
That did not happen.
Most did go through and some were slightly deflected, but a few bounced right back at very sharp angles. Rutherford said it was like firing a 15-inch shell from a Battleship's big gun at a sheet of tissue paper and having the shell bounce back and hit you:
No more plum pudding model.
There had to be a small, very dense hard core in the heart of the atom. And from the proportion of alpha particles bouncing back sharply, the size of the hard core of the atom could be worked out, about 10^-14 m for Gold.
That put us in the position of having to accept that electrons somehow orbited the nucleus and were locked in set orbits that they could only jump form one to the next by gaining or losing specific lumps of energy -- often, lumps of light called photons.
This is of course the Bohr model of the atom, an early Quantum model.
Beyond this, obviously there are many more details and complexities, but this should be enough for an early relatively simple picture of what is going on in Chemistry. And a lot of physics besides, too.
Let's see if we can discuss further and refine our thoughts. END